IN ADULT HEMODIALYSIS PATIENTS WITH MODERATE-TO-SEVERE CKD-ASSOCIATED PRURITUS (CKD-aP),
KORSUVA provides significant
CKD-itch relief
KORSUVA DEMONSTRATED PROVEN EFFICACY


KORSUVA produced clinically meaningful reductions in CKD-itch:
- The efficacy of KORSUVA was evaluated in two randomized, multicenter, double-blind, placebo-controlled trials (KALM-1 and KALM-2) that enrolled a total of 851 subjects 18
years of age and older undergoing HD who had moderate-to-severe pruritus - In each trial, efficacy was assessed based on the proportion of subjects achieving a
4-point or greater improvement (reduction) from baseline in the weekly mean of the daily 24-hour WI-NRS score at week 12 - Mean baseline WI-NRS scores were 7.1 and 7.2 in KALM-1 and KALM-2, respectively
KORSUVA PROVIDED RAPID AND SUSTAINED RELIEF
Itch reduction was seen by week 4 and sustained through week 12

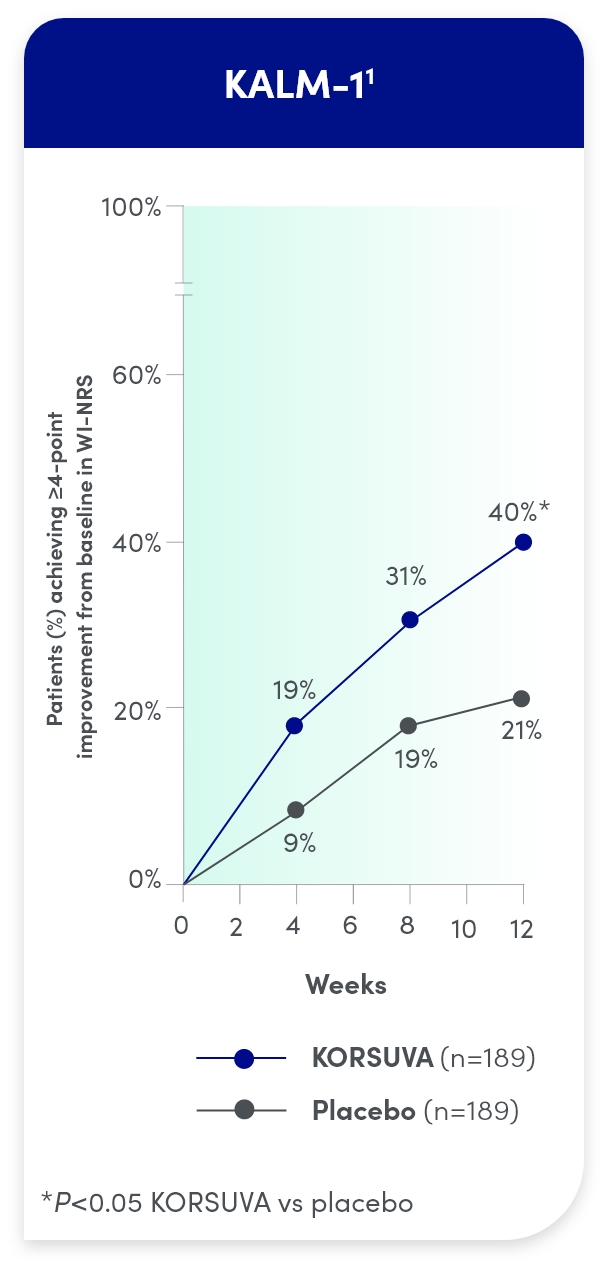
- Test for statistical significance was not prespecified at weeks 4 and 8 in KALM-1

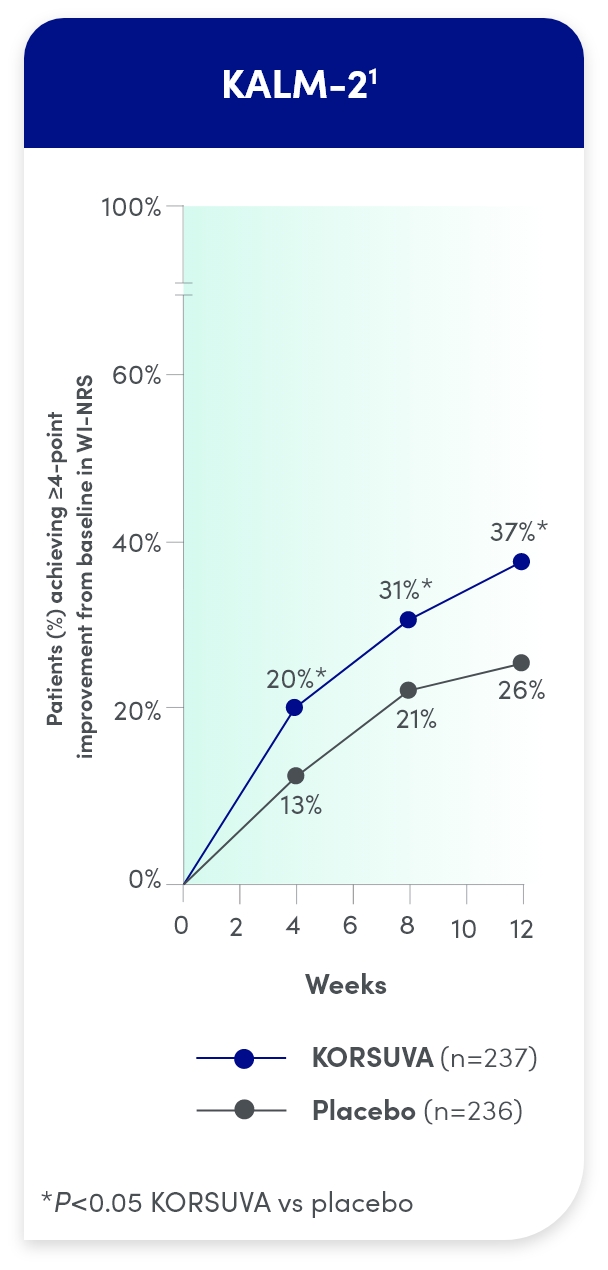
- Significantly more KORSUVA patients achieved a ≥4-point improvement in itch intensity at
12 weeks than placebo patients
WORST ITCHING INTENSITY NUMERICAL RATING SCALE (WI-NRS)


- WI-NRS is a validated scale that measures patient-reported itch intensity over a 24-hour period1,2
- It is a numerical rating ranging from 0 (“no itch”) to 10 (“worst itch imaginable”)
References: 1. Data on file as of August 2020. Cara Therapeutics. 2. Vernon M, Ständer S, Munera C, et al. Clinically meaningful change in itch intensity scores: An evaluation in patients with chronic kidney disease–associated pruritus. J Am Acad Dermatol. 2021;84(4):1132–1134. doi:10.1016/j.jaad.2020.06.991


