KORSUVA has convenient administration
- Recommended dosage is 0.5 mcg/kg based on patient dry body weight
- Administer KORSUVA by intravenous bolus injection into the venous line of the dialysis circuit at the end of each hemodialysis session
- KORSUVA may be given either during or after rinse back of the dialysis circuit
- KORSUVA can be administered up to 4 hours after syringe preparation
- KORSUVA is removed by the dialyzer membrane and must be administered after blood is no longer circulating through the dialyzer
- If the dose is given after rinse back, administer KORSUVA into the venous line followed by at least 10 mL of normal saline flush
KORSUVA DOSING SCHEDULE
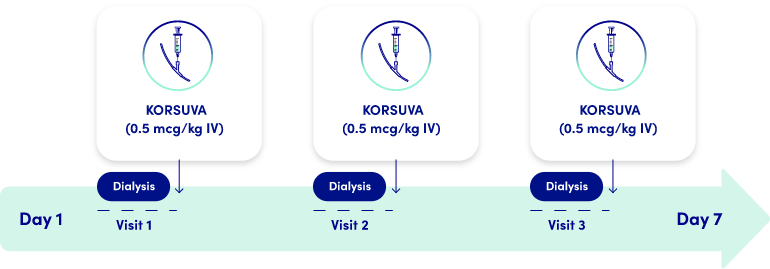
- If a regularly scheduled hemodialysis treatment is missed, resume KORSUVA at the end of the next treatment
KORSUVA DOSING
Use the calculator or table below to determine the recommended dosage for your patients.
Total injection volume (mL) = patient target dry body weight (kg) x 0.01, rounded to the
nearest tenth (0.1 mL).
| KORSUVA injection volumes based on target dry body weight | |
|---|---|
| Target dry body weight range (kg) | Injection volume (mL)* |
| 36–44 | 0.4 |
| 45–54 | 0.5 |
| 55–64 | 0.6 |
| 65–74 | 0.7 |
| 75–84 | 0.8 |
| 85–94 | 0.9 |
| 95–104 | 1.0 |
| 105–114 | 1.1 |
| 115–124 | 1.2 |
| 125–134 | 1.3 |
| 135–144 | 1.4 |
| 145–154 | 1.5 |
| 155–164 | 1.6 |
| 165–174 | 1.7 |
| 175–184 | 1.8 |
| 185–194 | 1.9 |
| 195–204 | 2.0 |
Total injection volume (mL) = patient target dry body weight (kg) x 0.01, rounded to
the nearest tenth (0.1 mL). To convert to lbs, total injection volume (mL) = patient
target dry body weight (lbs)/2.2 x 0.01. For patient target dry body weight outside of
the ranges in this table, use either of these formulas.
STORING AND PREPARING KORSUVA
- KORSUVA can be stored at room temperature
- Do not mix or dilute KORSUVA prior to administration
- KORSUVA is supplied in a single-dose vial. Discard any unused product


