CKD-aP is prevalent
MANY HEMODIALYSIS (HD) PATIENTS REPORTED SUFFERING FROM CKD-aP1
In the Dialysis Outcomes and Practice Patterns Study (DOPPS)*:
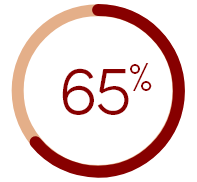
of HD patients in
the U.S. reported that
they suffered from CKD-aP†
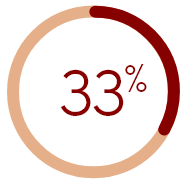
of HD patients in
the U.S. were moderately
to extremely bothered by itch
PATIENTS MAY NOT ALWAYS REPORT THEIR ITCHING2
In DOPPS‡:
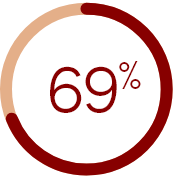
of medical directors
underestimated the prevalence of
CKD-aP in their dialysis facilities
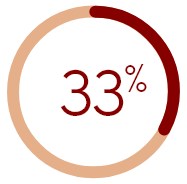
of U.S. HD patients who were nearly
always or always bothered by itching did
not report it to any healthcare provider
*Phases 4–6 of DOPPS (a prospective cohort study) included 34,694 HD patients in 21 countries from 2009–2018; 23,264 patients were eligible for analysis of the primary outcome. The primary clinical outcome was time to all-cause mortality; dialysis-related outcomes included withdrawal from dialysis and missed HD sessions; patient-reported outcomes included measures of health-related quality of life, depression, and sleep quality.
†Figure calculated based on total patients reporting being somewhat to extremely bothered by itching.
‡Phases 1–5 of DOPPS (a prospective cohort study) included 51,062 HD patients in 21 countries from 1996–2015; 35,452 patients answered the question regarding how bothered they were by itchy skin in a questionnaire and were included in this analysis.
References: 1. Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical,
dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med.
2020;3(1):42–53. doi:10.1016/j.xkme.2020.08.011 2. Rayner HC, Larkina M, Wang M, et al.
International comparisons of prevalence, awareness, and treatment of pruritus in people on
hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. doi:10.2215/CJN.03280317


